An FDA-Approved Topical Gel
HYFTOR® is a clear gel that contains 0.2% of the prescription medication sirolimus (an mTOR inhibitor). It is the first and only topical medication approved by the Food and Drug Administration (FDA) to treat facial angiofibroma in tuberous sclerosis complex (TSC) in adults and children 6 years of age and older.
Talk to Your Doctor about HYFTOR®!

Application
HYFTOR® should be applied to the skin of the face affected with angiofibroma twice daily, in the morning and at bedtime. The maximum recommended daily dosage is:
- Approximately 3/4 inch of gel (600 mg) for pediatric patients 6 to 11 years of age
- Approximately 1 inch of gel (800 mg) for adults and pediatric patients 12 years of age and older
HYFTOR® is only for topical use on the area of the face affected with angiofibroma. The skin being treated with HYFTOR® should not be covered with bandages, dressings, or wraps.
- Wash your hands before and after applying HYFTOR®
- Tell your healthcare provider if the treated skin area does not improve within 12 weeks of treatment
Results
In a clinical trial, improvement was seen at 12 weeks.
- 23% of patients had their Facial Angiofibroma assessed as “improved” or “markedly improved” after 12 weeks
- An assessment of “improved” was defined as at least a 50% reduction in size and a 2-level reduction in redness, and an assessment of “markedly improved” was defined as at least a 75% reduction in size and a 3-level reduction in redness
- 62 patients participated in the trial (including 25 patients between 6 and 18 years of age) The patients applied either HYFTOR® or a placebo (a gel containing no medication) to their faces twice daily for 12 weeks
- Results were reported at the end of 12 weeks. The improvement in size and redness of Facial Angiofibromas was assessed by the investigator (live assessment) using the subjects’ original baseline photographs as reference
Before and After Photos of Clinical Results
Individual results may vary.
Before
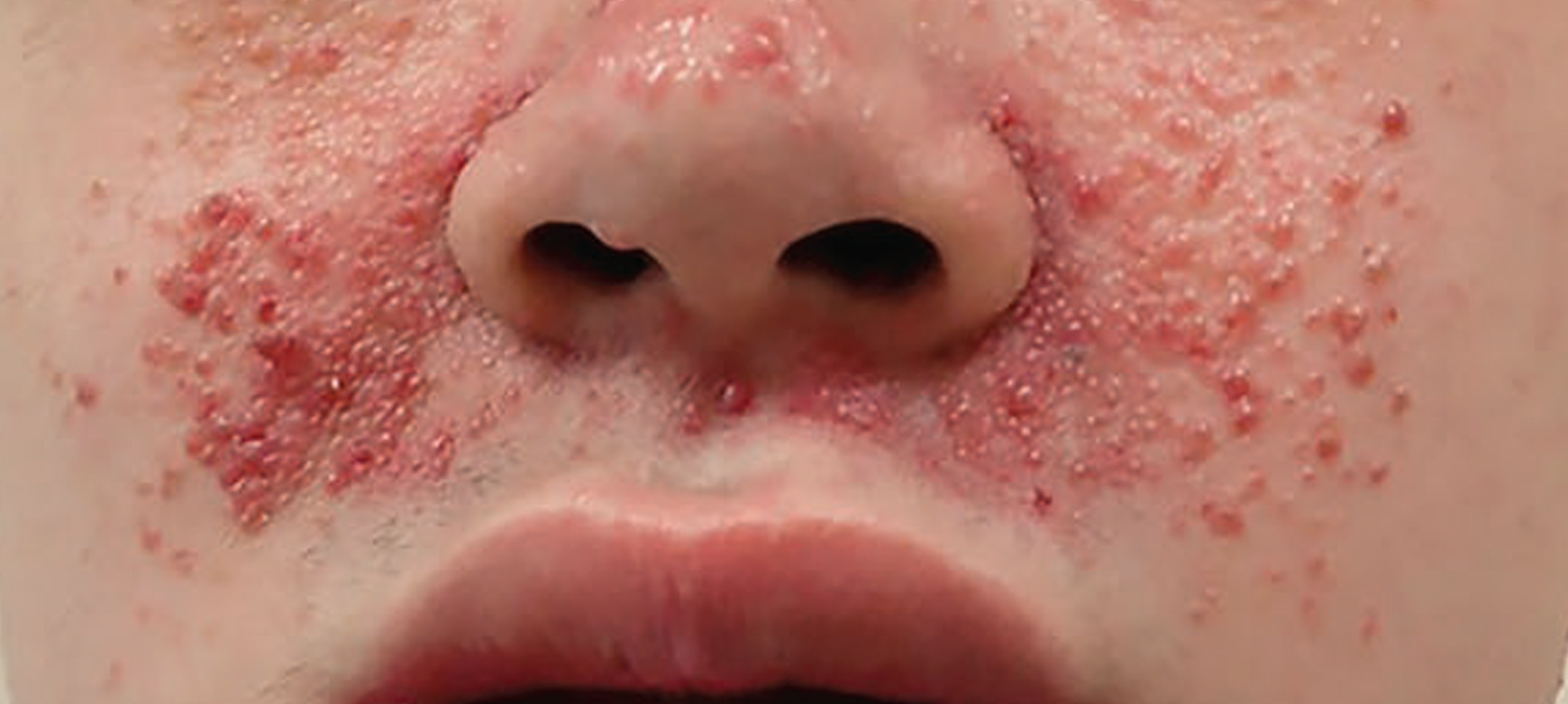
After 12 weeks
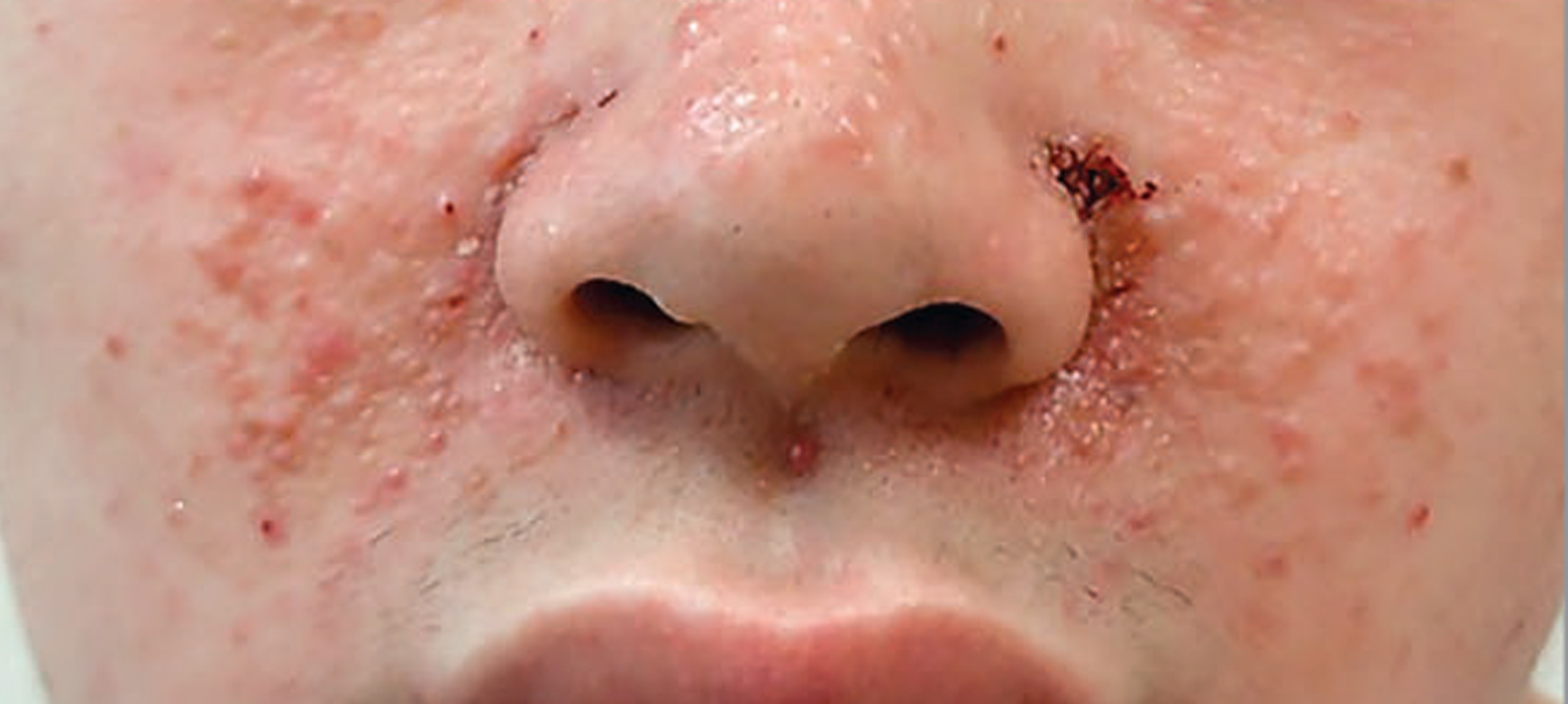
JAMA Dermatology. 2018;154(7):781-788. © 2018 American Medical Association. All rights reserved.
Side Effects
The most common side effects, occurring in 1% or more of patients treated with HYFTOR®, are:
- Dry skin
- Application site irritation
- Itching
- Acne
- Acne-like rash
- Eye redness
- Skin bleeding
- Skin irritation
These are not all the side effects associated with HYFTOR®. Please see additional Important Safety Information below and the Patient Information for more information. Before you start using HYFTOR®, you and your doctor should talk about all of your medical conditions, medicines you take, and skin products you use.
To report suspected side effects, contact Nobelpharma America, LLC, at 1-877-375-0825. You may also contact the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
Important Safety Information
What is HYFTOR®? HYFTOR® is a prescription medicine that is used on the skin (topical) to treat adults and children 6 years of age and older with a type of noncancerous tumor called angiofibroma on your face caused by the genetic condition tuberous sclerosis. It is not known if HYFTOR® is safe and effective in children under 6 years of age.
Important: HYFTOR® is for use on the skin only (topical use). Do not use HYFTOR® in your mouth, eyes, or vagina.
